72 100 As A Percent
Introduction
Madam Chairwoman, Ranking Member Burgess, and Members of the Subcommittee, I am Dr. Janet Woodcock, Director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA or the Agency), which is part of the Department of Health and Man Services (HHS).
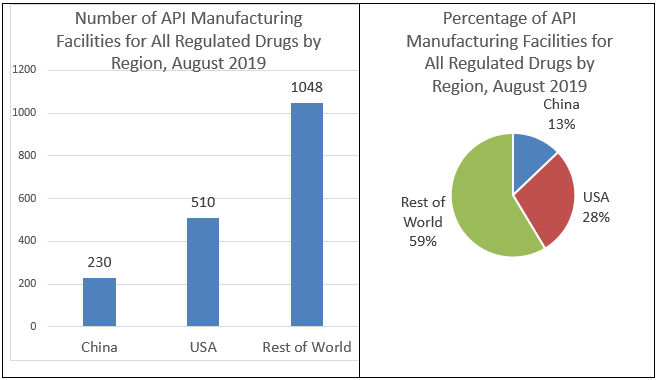
The U.s., through its investment in biomedical research, has become a world leader in drug discovery and development, but is no longer in the forefront of drug manufacturing. Historically, the product of medicines for the U.S. population has been domestically based. However, in recent decades, drug manufacturing has gradually moved out of the United States. This is particularly true for manufacturers of active pharmaceutical ingredients (APIs), the actual drugs that are then formulated into tablets, capsules, injections, etc. As of Baronial 2019, only 28 percent of the manufacturing facilities making APIs to supply the U.S. market were in our country. By dissimilarity, the remaining 72 pct of the API manufacturers supplying the U.S. market were overseas, and xiii pct are in Red china. (See Effigy 1) FDA's data prove that the number of registered facilities making APIs in China more than than doubled betwixt 2010 and 2019.
Figure 1: Manufacturing Sites of APIs for U.South. Market by Country or Region,
August 2019
While at that place are many reasons for this shift, underlying factors that are ofttimes cited include the fact that most traditional drug production processes require a large manufacturing plant site, oftentimes have environmental liabilities, and can utilize a low-cost labor force. A 2009 paper by the World Bank, "Exploratory Study on Active Pharmaceutical Ingredient Manufacturing for Essential Medicines," stated that if a typical Western API company has an boilerplate wage alphabetize of 100, this alphabetize is as depression as 8 for a Chinese company and 10 for an Indian visitor.[one] Red china has lower electricity, coal, and water costs. Chinese firms are also embedded in a network of raw materials and intermediary suppliers, and so take lower aircraft and transaction costs for raw materials. They as well face fewer environmental regulations regarding ownership, handling, and disposing of toxic chemicals, leading to lower directly costs for these firms. FDA's 2011 study, "Pathway to Global Production Safety and Quality," noted that both Mainland china and India enjoy a labor toll advantage and that API manufacturing in India can reduce costs for U.S. and European companies by an estimated 30 percentage to 40 percent.[2]
Using traditional pharmaceutical manufacturing technology, a U.S.-based company could never offset the labor and other cost advantages that Communist china enjoys simply past achieving higher productivity. However, FDA believes that advanced manufacturing technologies could enable U.Due south.-based pharmaceutical manufacturing to regain its competitiveness with China and other foreign countries, and potentially ensure a stable supply of drugs critical to the health of U.S. patients. Avant-garde manufacturing is a collective term for new medical production manufacturing technologies that can ameliorate drug quality, address shortages of medicines, and speed time-to-market place. Every field has a different set of product techniques that are considered advanced. Examples of some cross-cutting advanced manufacturing technologies include continuous manufacturing and 3D printing. Advanced manufacturing technology, which FDA supports through its Emerging Technology Program (ETP), has a smaller facility footprint, lower environmental touch on, and more than efficient apply of human being resource than traditional engineering, every bit will be explained later in this testimony.
The pharmaceutical sector relies heavily on foreign sourcing for disquisitional components, materials, and finished products, as identified in the U.Southward. Department of Commerce'south Part of Technology Evaluation's 2011 report, "Reliance on Foreign Sourcing in the Healthcare and Public Health (HPH) Sector: Pharmaceuticals, Medical Devices and Surgical Equipment."[3] Yet, use of strange-sourced materials creates vulnerabilities in the U.S. drug supply. For instance, in August 2018 FDA issued an alert that a Chinese API manufacturer, Sichuan Friendly Pharmaceutical Co. Limited, was recalling certain lots of porcine thyroid API due to inconsistent quality of the API.[4] This thyroid API comes from porcine (grunter) thyroid glands and is used to brand a medicine to treat hypothyroidism (underactive thyroid). FDA laboratory testing confirmed that the Sichuan Friendly API had inconsistent levels of active ingredients and should not be used to manufacture or compound drugs for patient utilise. Risks associated with over- or undertreatment of hypothyroidism could result in permanent or life-threatening agin health consequences.
In December 2015, FDA alerted drug compounders that certain lots of baclofen API manufactured by Chinese manufacturer Taizhou Xinyou Pharmaceutical & Chemical Co., Express might exist at risk for contagion with particulates and should not be used to compound sterile injectable drugs. Taizhou manufactures APIs for repackagers and distributors, some of which sell these products to compounding facilities in the United States.[five]
FDA contacted Taizhou and the company confirmed that, due to the level of controls in the manufacturing procedure, the baclofen API information technology manufactures was not suitable for use in injectable drugs. FDA recommended that no baclofen API from Taizhou be used to manufacture or compound any injectable drugs. The affected API potentially could have posed serious safety risks for U.S. patients who used or received injectable drug products compounded with the affected baclofen, peculiarly when administered direct into the spine (intrathecally). There was also a potential take a chance that the baclofen API might have been contaminated by endotoxin or microorganisms.
Today, I would like to share CDER's data about the location of API manufacturing facilities in Red china, the United States, and the rest of the world; discuss the implications for national security; and explain how avant-garde manufacturing can increase the security and reliability of the U.S. drug supply.
Explanation of CDER'due south Data and its Limitations
From a national security perspective, information technology is useful to look at the locations of facilities for three sets of drugs:
- All drugs on the U.Southward. market place, including brand and generic drugs under approved applications, over-the-counter (OTC) drugs, and compounded medications.
- Drugs on the Earth Health Organization (WHO) Essential Medicines Listing that are marketed in the United States.
- Drugs on the medical countermeasures (MCM) lists. These include drugs we would use to counter biological, chemical, nuclear, or radiation threats and influenza.
CDER maintains a Site Catalog ("Catalog") of all manufacturing facilities making drugs for the U.S. market, either through an approved application or that take registered and listed to supply drugs for the U.S market. This includes suppliers for API, finished dosage forms (FDF), or both. The APIs manufactured in these facilities may be used in prescription drugs (brand or generic), OTC drugs, and compounded drugs.
Information bachelor to CDER have several limitations, including the post-obit:
- Facilities listed in the Catalog may or may not exist producing APIs. Including a facility in an awarding or the registration and listing process does not require a facility to produce API. Producing an API at the facility, or not producing information technology, is a business organisation decision made by the company.
- Manufacturers are not required to report to FDA whether they are actually producing an API at a facility, and if they are, the volume they are producing.
- APIs made in listed facilities may exist used in drugs for both the U.Southward. and other markets, and some APIs distributed in the United States are afterwards formulated into FDF that are and then exported.
- Some FDF applications list more than than ane API supplier in the application. FDA has no visibility into which API supplier an FDF manufacturer uses at any given time.
- CDER has express data near API suppliers for products that do non need an canonical application from FDA to exist marketed, such as compounded and OTC monograph drugs. API suppliers for such products may not register their facility with FDA if they are sending cloth to a drug product manufacturer outside the U.s.a. to make the FDF, which is and so sold in the Us.
- Information in the Itemize is continually being updated. The analysis presented below is based on August 2019 listings and represents a snapshot at a point in time.
These limitations mean that, although CDER can describe the locations of API manufacturing facilities, we cannot determine with any precision the book of API that China is really producing, or the book of APIs manufactured in Communist china that is entering the U.Due south. market, either direct or indirectly past incorporation into finished dosages manufactured in Communist china or other parts of the earth.
API Manufacturing Facilities for All Regulated Drug Products
CDER'southward assay shows that overall, China has only a modest percentage of the facilities able to produce APIs for the U.Due south. market. For all regulated drugs, Prc has 230 (xiii percent) of the API manufacturing facilities, while the United States has 510 (28 percent), and the residuum of the world has 1048 (59 percentage). "All regulated drugs" includes prescription (make and generic), OTC, and compounded drugs.(Meet Figure two) Notwithstanding, the percentages of APIs produced at these facilities may differ, and as mentioned above, cannot be determined from the data available to FDA.
Effigy ii: Number and Percentage of API Manufacturing Facilities for All Drugs by Region, Baronial 2019
API Manufacturing Facilities for WHO Essential Medicines on the U.S. Market place
The 2019 WHO Essential Medicines List comprises 461 drugs that have been selected by the WHO Expert Committee to come across the most important needs in a health organization. This list includes application and non-application products beyond a wide range of therapeutic categories such as coldhearted, antibacterial, antidepressant, antiviral, cardiovascular, anti-diabetic, and gastrointestinal agents.
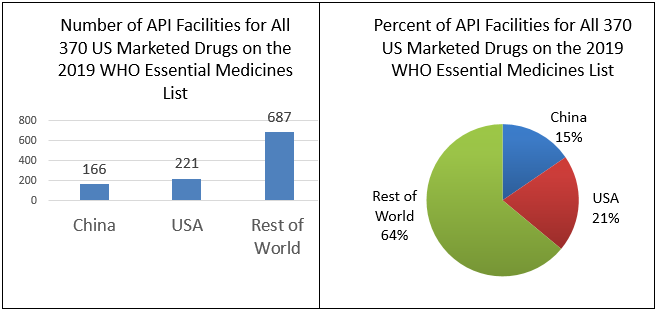
FDA matched 370 of the drugs on the WHO Essential Medicines Listing with products listed for the U.S. marketplace and determined the location of the facilities used to make their APIs.[6] FDA data prove that at that place is a total of 1,079 API facilities worldwide that make the 370 drugs on the WHO list that are marketed in the U.S. Of these, 166 (xv%) are in Cathay, 221 (21%) are in the United States, and 687 (64%) are in the residual of the world.(See Figure iii)
Figure 3: Number and Percentage of API Facilities for the 370 U.Due south. Marketed Drugs on the 2019 WHO Essential Medicines Listing
FDA adamant that in that location are three WHO Essential Medicines whose API manufacturers are based simply in China. The iii medicines are: capreomycin and streptomycin, both indicated to treat Mycobacterium tuberculosis; and sulfadiazine, used to treat chancroid and trachoma.
The distribution of API facilities worldwide varies from drug to drug and may differ from the patterns for all drugs or WHO Essential Medicines Listing Drugs.
API Manufacturing Facilities for Medical Countermeasures
FDA maintains a list of drugs that are used as medical countermeasures (MCMs) against threats in four categories: biological threats, chemic threats, flu, and radiation threats. Many of these drugs are contained in strategic drug stockpiles, including the Nation's Strategic National Stockpile, the Nation's largest supply of potentially life-saving pharmaceuticals and medical supplies for utilize in a public health emergency severe enough to cause local supplies to run out.
For APIs for 14 drugs in the biological threat category, China has 37 facilities, the United States has 19, and the rest of the world has 117. China has just six of the facilities producing APIs for the 10 drugs in the chemical threat category, versus 24 in the United states of america and 52 in the rest of the world. (Encounter Table i)
| MCM Blazon (# products) | U.Southward. API Sites # (%) | China API Sites # (%) | Other Strange API Sites # (%) |
|---|---|---|---|
| Biological (14) | 19 (11%) | 37 921% | 117 (68%) |
| Chemical (10) | 24 (29%) | six (seven%) | 52 (64%) |
| Influenza (3) | 2 (11%) | 0 (0%) | sixteen (89%) |
| Radiations (vii) | 13 (46%) | 0 (0%) | xv (54%) |
Tabular array ane: Number and Percent of API Manufacturing Sites for MCM Drugs for Use Against Biological, Chemical, and Radiation Threats, and Flu, August 2019
China has none of the facilities making APIs for medicines to forestall or care for flu versus ii in the The states and 16 in the rest of the world. Communist china also has none of the facilities producing APIs for radiation threats. The The states has thirteen of these facilities versus 15 in the residue of the world.
Ciprofloxacin and doxycycline are two drugs considered critical as MCMs and used to treat anthrax and plague. As shown in Tabular array 2 below, the United States has i facility for ciprofloxacin, versus iii in Red china and 21 in other strange countries. The United States has fewer facilities than China or other foreign countries for doxycycline. (Run across Tabular array two)
| MCM | # (%) U.S. API Sites | # (%) China API Sites | # (%) Other Foreign API Sites |
|---|---|---|---|
| Ciprofloxacin | 1 (4%) | 3 (12%) | 21 (84%) |
| Doxycycline | 2 (eighteen%) | 3 (27%) | 6 (55%) |
Tabular array 2: Number and Pct of API Manufacturing Sites for Ciprofloxacin and Doxycycline by Region, August 2019
Implications for National Security
The security of the nation'south drug supply rests on three main factors: freedom from dependence on foreign sources of API, the resilience of our domestic manufacturing base, and the reliability of the facilities that make products for the U.S. market place.
Dependence: How dependent are we on Prc, India, or other countries for the APIs used in drugs produced for patients in the United states of america? How has this dependence changed over fourth dimension?
The number of Chinese facilities producing APIs for the U.S. market has increased over the past decade, every bit function of a massive move of pharmaceutical production offshore. This movement is being driven past the pharmaceutical manufacture'southward desire for cost savings and less stringent environmental regulations. Absent any intervention, FDA believes that this tendency is likely to continue.
However, information available to FDA do not enable usa to calculate the book of APIs being used for U.S.-marketed drugs from China or India, and what per centum of U.S. drug consumption this represents. Every bit mentioned higher up, we do non know whether Chinese facilities are actually producing APIs, how much they are producing, or where the APIs they are producing are existence distributed worldwide, including in the Us.
Resilience: How resilient is the U.Southward. manufacturing base of operations? How quickly could U.S.-based manufacturers increase their product of APIs to run into domestic demand if China or India, or another state, ceased supplying the United States, particularly for drugs on the WHO Essential Medicines listing or a subset that is widely used by the U.S. population?
To reply this question, FDA would need to know:
- how much unused chapters exists in the U.Due south. manufacturing base of operations for APIs;
- how much additional API this capacity could supply within a given time menstruum;
- how far this capacity would get in filling the gap between U.Southward. patients' needs and the amount available if China or India, or some other state, were to reduce or stop the supply to the U.S. market; and
- how long would it take to increase production enough to meet patients' needs, and whether the financial investment would be sustainable for the pharmaceutical industry.
Since we do not currently know whether API manufacturing facilities are really producing the drug, or in what book, or what portion of U.S. drug consumption is dependent on APIs from Cathay or India, or another country, we cannot perform a reliable gap analysis.
Even if we could estimate the potential API shortfall and available production capacity, pharmaceutical companies brand business decisions nigh whether to produce a drug product, including an API, and FDA does non have the power to tell them to make a drug. This provides additional dubiousness in assessing the potential responsiveness of the U.South. manufacturing base to a crisis triggered by another land's withdrawal.
Reliability: How reliable is the manufacturing base that produces APIs for the U.S. market?
FDA recently analyzed 163 drugs that went into shortage during the five-year menses from 2013 to 2017 and establish that quality problems were responsible for the shortages 62 pct of the time.[vii] These shortages can worsen patients' health outcomes by causing delays in treatment or changes in treatment regimens, such as substituting less-effective or well-tolerated medicines when a drug of choice is not bachelor.
In looking for means to ensure Americans' access to a supply of safe and effective drugs, nosotros need to consider all three dimensions of the problem.
Summary of National Security Findings
FDA's data shows that overall, the number of China'south API facilities is somewhat smaller than the United States, but comparable in size and growing. Yet, because of the limitations of available data, we cannot assess the extent of U.South. dependence on Cathay. For instance, we exercise not have information well-nigh the book of API existence produced in China or even in the United States, or how much of Prc's API output reaches the U.Southward. market through other countries.
Similarly, we practice not accept information that would enable us to assess the resilience of the U.S. manufacturing base, should it be tested past China'due south withdrawal from supplying the U.S. market. We do know that the U.S. drug supply is being compromised by drug shortages, in near cases triggered by manufacturing quality problems by U.Southward.-based as well as foreign producers.
Avant-garde Manufacturing Offers a Multi-dimensional Solution
Avant-garde manufacturing is the use of innovative technology to meliorate products and processes. Although widely used in some other industries, such equally automotive, aerospace, and semiconductors, advanced manufacturing is at present only kickoff to be used by pharmaceutical companies. For API and/or FDF manufacturing, new technologies include "continuous manufacturing" (CM), wherein the finished drug production is produced as a continuous stream, as opposed to traditional batch manufacturing where breaks or stops exist between different processing steps. In some examples of advanced pharmaceutical manufacturing, production tin be continuous from chemical synthesis of the active ingredient through production of the tablets or other dosage forms.
Avant-garde manufacturing offers many advantages over traditional pharmaceutical manufacturing, and if the United States invests in this engineering, it can be used to reduce the Nation's dependence on foreign sources of APIs, increase the resilience of our domestic manufacturing base, and reduce quality issues that trigger drug shortages or recalls. For example:
- Production quality tin be precisely controlled with modern automation and command systems and tin can be closely monitored during production past using loftier-resolution analytics.
- Loftier technology, reckoner-controlled production facilities are improve able to rapidly respond to changes in demand considering they typically practise non have the equipment scale-upwardly bug associated with traditional methods and tin be capable of seamlessly producing a variety of dosages and even dosage forms.
- Advanced manufacturing platforms also have a much smaller footprint than traditional manufacturing platforms, and the equipment can exist made portable so that it can be moved closer to markets, reducing the need for transcontinental shipping of components.
- Medicines can be produced at lower toll than past traditional methods.
- Environmental impact of manufacturing is significantly reduced.
By supporting the growth of advanced manufacturing in the The states, nosotros can reduce our dependence on China and other overseas manufacturers for APIs as well every bit better the resilience and responsiveness of our manufacturing base of operations and reduce drug shortages.
FDA'due south advanced manufacturing initiative is fostering this growth in several ways.
Emerging Technology Program (ETP)
The ETP, launched in late 2014, encourages and supports the adoption of innovative technology to modernize pharmaceutical development and manufacturing through close collaboration with manufacture and other relevant stakeholders starting from early engineering development.
To reduce barriers to entry for advanced manufacturing, the Emerging Technology Team (ETT) provides a gateway for the early (pre-submission) give-and-take of innovative technologies and approaches, fifty-fifty earlier a candidate drug is identified. The ETT supports the entry, assessment, and lifecycle management of advanced manufacturing at CDER. It provides bailiwick matter experts and fosters coordination within CDER and FDA's Office of Regulatory Diplomacy (ORA) for precedent-setting issues regarding quality and good manufacturing practices. ETT serves as a hub for identification of application-driven regulatory and research needs and provides strategic input for supporting avant-garde manufacturing innovation. Based on ETT efforts in continuous manufacturing, CDER's Office of Pharmaceutical Quality (OPQ) published a draft guidance, "Quality Considerations for Continuous Manufacturing" of solid oral dosage forms in early 2019.[8]
Under this plan, CDER has approved five drug applications utilizing continuous manufacturing for FDF manufacturing, and the kickoff awarding utilizing 3-D press technologies. Currently, these drugs are being made in the United States, and one drug is being made both in the Us and in the U.k..
Regulatory and Policy Initiatives
The adoption of advanced manufacturing technologies may pose a challenge to the current regulatory framework, because most regulations were developed based on traditional batch manufacturing methods under a unified pharmaceutical quality arrangement. As a effect, FDA has launched an endeavor to identify and implement needed changes in the regulatory construction. For example, new policy and regulatory topics related to emerging technologies include the direction of data-rich environments, the evolving concepts of process validation for advanced manufacturing systems, and the regulatory oversight of postal service-approval changes for such systems. Furthermore, CDER, in collaboration with the Biomedical Advanced Enquiry and Development Authority (BARDA), is working on a strategy and new regulatory framework to develop and implement miniature, mobile manufacturing platforms ("Chemist's on Demand") for industry of essential drugs virtually or at the point of intendance.
FDA actively engages with stakeholders in industry, academia, and other regulatory agencies to identify and address regulatory hurdles to adoption of advanced manufacturing. For case, CDER, in partnership with FDA'southward Center for Biologics Evaluation and Research (CBER), is leading the International Quango for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) endeavor to develop the Q13 guideline on continuous manufacturing of drug substances and drug products for both small-molecule and biological products, which volition assist to reach global regulatory harmonization.
Intramural and Extramural Research
Laboratories in CDER'south OPQ actively conduct advanced manufacturing enquiry and invest in equipment, facilities, and personnel with expertise to investigate these topics. OPQ has established the Heart of Excellence for Manufacturing Science and Innovation to coordinate internal advances in manufacturing inquiry for both small molecules and biologics. OPQ publishes and leads research on continuous manufacturing, advanced analytics for process controls, and modeling and simulation. OPQ also provides training for assessment and inspection personnel.
Extramurally, OPQ awards research grants and contracts for advanced manufacturing and emerging technologies. OPQ also participates in consortia with academia and industry to place new areas for research in advanced manufacturing. Participation in these intramural and extramural research efforts occurs with potent alignment and coordination between assessment, policy, and surveillance offices. This ensures that resources are allocated to projects that provide practical approaches to regulating innovative technologies.
On behalf of FDA, I would similar to thank Congress for having the foresight to provide resources to back up our efforts to help the pharmaceutical manufacture as it makes a transition from traditional manufacturing methods to the use of advanced technologies. These new tools and methods have the potential to reinvigorate our pharmaceutical manufacturing base and repatriate it from overseas. This will assistance to ensure that Americans accept a secure and reliable supply of medicines in the future, every bit well every bit contribute to our economic system.
Promoting Domestic Manufacturing
In FY19, Congress approved appropriations to promote domestic manufacturing with the intent that FDA advance modern drug and biological product technologies. The spending plan for these additional resources builds on the CDER strategic goals to improve overall staff agreement and expertise in avant-garde manufacturing by expanding support of these innovative technologies in cess, policy, surveillance, and research, also as past making programmatic improvements. In add-on, this funding is used to reinforce extramural outreach with stakeholders via planned technology forecasting activities with the National Academies of Sciences, Engineering, and Medicine and other forums.
Conclusion
The increasing number of API manufacturing sites in China and other countries suggests that the United States' reliance on Chinese and other foreign sources of API is growing. FDA has been working diligently in collaboration with industry and other federal agencies to ensure our reliance of foreign manufacturing does not pose a national security risk. While FDA cannot tell industry where they tin can and cannot manufacture APIs, we can piece of work with manufacture to utilise new technologies and new manufacturing methods to farther incentivize domestic product of drugs and APIs. These new ways of making drugs could, with the proper strategies, revitalize pharmaceutical manufacturing in the United States.
Footnotes
6. Some of the 461 drugs on the 2019 WHO Essential Medicines List were excluded from the analysis considering they are non regulated by CDER. The Listing includes products regulated by the Center for Biologics Evaluation and Inquiry (CBER), such as cholera vaccine and anti-rabies immunoglobulin, and products regulated by the Center for Devices and Radiological Health (CDRH), such as diaphragms and condoms.
7. U.S. Food and Drug Assistants, "Drug Shortages: Root Causes and Potential Solutions," October 2019.
72 100 As A Percent,
Source: https://www.fda.gov/news-events/congressional-testimony/safeguarding-pharmaceutical-supply-chains-global-economy-10302019
Posted by: hebertidentradmus1951.blogspot.com



0 Response to "72 100 As A Percent"
Post a Comment